 About the Company:
About the Company:
Anlon Healthcare Ltd is a chemical manufacturing company engaged in manufacturing of; (i) high purity advance pharmaceutical intermediates which serves as raw material/ key starting material in the manufacturing of active pharmaceutical ingredients; and (ii) active pharmaceutical ingredients (“APIs”) which serves as a raw material for pharmaceutical formulations in preparation of various type of Finished Dosage Formula (“FDF”) such as tablet, capsules, ointment, syrup etc, ingredients in nutraceuticals formulations, personal care products and animal health products.
Company’s products spans across the family of pharmaceutical intermediates, active pharmaceutical ingredients, nutraceutical APIs and ingredients for personal care and veterinary API.
Company is one of the few manufacturers of loxoprofen sodium dihydrate in India, which is a notable API widely used in treatment of pain/inflammation association with conditions including rheumatoid arthritis, osteoarthritis, lower back pain, frozen shoulder, neck-shoulder-arm syndrome, tooth pain or after surgery, injury or tooth extraction
In addition to the manufacturing of Pharma Intermediate and APIs in accordance with various domestic and international standards, we have recently started undertaking custom manufacturing services for complex or novel chemical compounds, tailoring the manufacturing process to meet specific customer requirements, including producing chemicals with purity levels that exceed industry standards.
Company’s domain knowledge and expertise enables us to reduce existing impurities and employ appropriate processes to achieve the desired level of purity.
Company also undertakes API development, preparation and filing of Drug Master File (“DMF”) in the Indian and global markets as per the pharmacopeia requirements of our customers and regulatory agencies.
For the Fiscal 2025, Fiscal 2024 and Fiscal 2023, Company manufactured and sold 338MT, 153 MT and 316 MT of API and Pharma Intermediates to 38, 39 and 48 customers.
Company’s Revenue derived from the pharma industry segments where our products have been used in various applications: (in Lakhs)
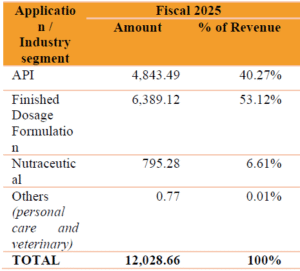
Company’s Customers:
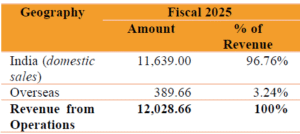
Company supplies products in both domestic and overseas markets to various pharmaceutical companies and third party dealers and distributors.
In addition to our domestic market sales in India, we have expanded our scale of operations and global footprint with customers in over 15 countries including Italy, Germany, South Korea, China, Argentina, Chile, Columbia, Mexico, Egypt, Turkey, Japan, Brazil, United Kingdom, United Arab Emirates etc. among others.
Company’s Revenue from Domestic and Exports:
Company’s Manufacturing Facility:
Company carry’s out manufacturing activities through our manufacturing facility situated at Survey No.36/2/P2, Near Bharudi Toll Plaza, Gondal Road NH27, Sadak Pipaliya, Rajkot, Gujarat which is spread across 5,059 sq. mtrs.
Approvals for Company’s Manufacturing Facilities:
Company’s Manufacturing Facility was recently audited and approved by Brazilian Health Regulatory Agency (“ANVISA-Brazil”) with zero discrepancy.
Further, Company’s Manufacturing Facility received the Accreditation of Foreign Manufacturer from Pharmaceuticals and Medical Devices Agency, Japanese Regulatory Authorities (“PMDA-JAPAN”) and was approved by National Medical Products Administration, China (“NMPA-China”).
Further, Company’s manufacturing units have been accredited by Good Manufacturing Practise (“GMP”) and GMP-WHO for APIs which ensures that our API products are in adherence to the quality standards appropriate to their intended use and are as per the product specification.
Further, the management systems of our Manufacturing Facility is compliant with ISO 9001:2015.
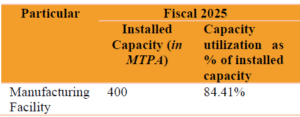
Company’s Installed Capacity & Capacity Utilization:
Management Team:
- Mr. Punitkumar R. Rasadia (Designation: Chairman and Managing Director)
- Mr. Meet Atulkumar Vachhani (Designation: Whole-time Director)
Objectives of the Issue:
Fresh Issue: (Rs.121 cr)
- Funding capital expenditure requirements for expansion of our Manufacturing Facility (“Proposed Expansion”).
- Full or part repayment and/or prepayment of certain outstanding secured borrowings (term loan) availed by our Company.
- Funding the working capital requirements of our Company.
- General corporate purposes.
Fund Utilization:
| Particulars | Amount (Rs. in Crores) |
|---|---|
| Funding capital expenditure requirements for Proposed Expansion | 30.71 |
| Full or part repayment and/or prepayment of certain outstanding secured borrowings secured borrowing (term loan) availed by our Company | 5.0 |
| Funding the working capital requirements of our Company | 43.15 |
Positives for the Company:
Strong product portfolio and scalable business.
- Company is one of the few manufacturers of loxoprofen sodium dihydrate in India, which is a notable API that is widely used in treatment of pain/inflammation association with conditions including rheumatoid arthritis, osteoarthritis, lower back pain, frozen shoulder, neck-shoulder-arm syndrome, tooth pain or after surgery, injury or tooth extraction.
- Company’s API products loxoprofen sodium dihydrate has been approved by regulatory authority of Brazil, Japan, China and our present position as one of the few manufacturers of loxoprofen sodium dihydrate in India, allows us to serve the customers of such jurisdiction.
High entry and exit barriers due to long customer approval cycles and strict product standards.
- Company is a manufacturer of Pharma Intermediates and APIs. Our manufacturing process involves multi-step production and purification processes to manufacture Pharma Intermediates and APIs.
- Further, given the nature of the application, our processes and products are subject to, and measured against established domestic and international standards and stringent specifications of customers.
- Customer acquisition involves a long process and gestation period is higher. Further, our Manufacturing Facility is regularly audited by our customers or their external consultants to ensure that we meet their quality and process standards.
- As a result of extensive experience of working with domestic and multinational customers across jurisdictions, we believe that we are well positioned to capitalise on our experience and expertise to generate and obtain repeat orders from our customers.
Company Plans to Increase our manufacturing capacity to focus on the growing demand of our core products.
- Currently company has one manufacturing facility situated at Rajkot, Gujarat, India for the production of our wide range of Products. Company’s total installed capacity is 400 MTPA. The Manufacturing Facility is spread over a land area of approximately 5,059 Sq.mts.
- Company intends to expand our manufacturing operations and production capacity by establishing a new manufacturing plant on Company’s owned freehold industrial land situated at survey number 42/1/p2/p2 , Village Pipaliya, Taluka Gondal, District Rajkot, Gujarat, India, admeasuring 4,958 sq.mts.
- The proposed new manufacturing plant shall be with an intermediate block and API block having an installed capacity of 700 MTPA, thus increasing the total production capacity. Company propose to utilize the additional capacity for manufacturing a range of existing as well as new Pharma Intermediaries and APIs.
Company Plans to Expand the Existing Product Portfolio.
- Company has consistently sought to diversify our portfolio of products which could cater to customers across segments, sectors, and geographies. We have enabled us to expand our product offerings from ten (10) commercial products in Fiscal 2018 to sixty-five (65) commercial products during Fiscal 2025.
- In accordance with this, while we seek to continue to strengthen our existing product portfolio, we intend to further diversify into products with prospects for increased growth and profitability. Company plans to continue to increase offerings in our current business segments as well as diversify into new products by tapping into segments which in the view of our management have attractive growth prospects.
Financials of the Company:
| (in Crores) | FY 23 | FY 24 | FY 25 |
|---|---|---|---|
| Revenue | 113.12 | 66.69 | 120.45 |
| Net Profit | 5.82 | 9.65 | 20.51 |
Valuation of Peer Group Companies:
| Company Name | Face Value | EPS | PE Ratio | RoNW | NAV |
|---|---|---|---|---|---|
| Anlon Healthcare Ltd | 10 | 6.38 | 14.26 | 25.51% | 0.73 |
| Kronox Lab Sciences Ltd | 10 | 6.91 | 26.18 | 28.26 | 24.28 |
| Acutaas Chemicals Ltd | 5 | 19.81 | 58.47 | 12.15% | 161.24 |
| Supriya Lifeciences Ltd | 2 | 23.35 | 29.27 | 18.86% | 123.85 |
IPO Details:
| Details | Info |
|---|---|
| Issue Opens on | 26th August 2025 |
| Issue Closes on | 29th August 2025 |
| Issue Price | Rs.86 -91 |
| Face Value | Rs.10 |
| Retail Category Allocation | 10 % |
| Minimum Lot | 164 Shares |
| Minimum Investment | Rs.14,924 |
| Issue Constitutes | 25.02 % |
| Issue Size | Rs.121 cr ($ 13 million) |
| Market Cap | Rs.484 cr ($ 56 million) |
| Listing at | NSE & BSE |
| Equity Shares Offered (Fresh) | 1,33,00,000 (Rs.121 cr) |
| Equity Shares Prior to the Issue | 3,98,51,500 |
| Equity Shares after the Issue | 5,31,51,500 (Rs.484 cr) |
IPO Valuation Parameters:
| Earnings Per Share (EPS) | Price To Earnings ratio (PE) | Return on Net Worth (RoNW) | Net Asset Value (NAV) | Debt Equity Ratio (D/E) |
|---|---|---|---|---|
| 6.38 | 14.26 | 25.51% | 20.18 | 0.73 |
Also Read: Complete List of NSE/BSE Holidays List>>
Important Dates:
| Finalization of Basis of Allotment | on or Before 1st September 2025 |
| Initiation of Refunds | on or Before 2nd September 2025 |
| Credit of Equity Shares: | on or Before 2nd September 2025 |
| Listing Date: | on or Before 3rd September 2025 |
| Company Contact Info: |
|---|
| Anlon Healthcare Ltd 101/102, Silvercoin Complex, Opp.Crystal Mall, Kalawad Road, Rajkot – 360 005, Gujarat, India. Telephone: +91 281 256 2538/39 E-mail: cs@anloncro.com; Website: www.anlon.in |
| Registrar to the Issue: |
|---|
| KFin Technologies Limited Selenium, Tower-B, Plot No- 31 and 32, Financial District Nanakramguda, Serilingampally, Hyderabad – 500 032, Telangana, India Telephone: +91 40 6716 2222 / 1800 309 4001 Email: ahl.ipo@kfintech.com Website: www.kfintech.com |



